What Does the First Quantum Number N Describe
Select the correct answer below. N is the first quantum number.

Different Orientations Of The First Four Orbital Shapes Image Credit Modified By Helen Klus Origin Quantum Mechanics Quantum Entanglement Teaching Chemistry
The azimuthal quantum number denotes the subshell of the electron.
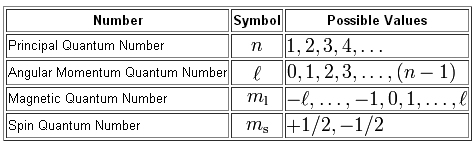
. What are the 4 quantum numbers describe each. Energy n angular momentum ℓ magnetic moment mℓ and spin ms. What do the first 3 quantum numbers indicate.
If n 1 there is only one possible value of l. To completely describe an electron in an atom four quantum numbers are needed. L is the secondquantum number.
The type of orbital the electron is. O The energy level of the orbital. The first quantum number describes the electron shell or energy level of an atom.
O The orientation in space of the orbital. For example in caesium Cs the outermost valence electron is in the shell with energy level 6 so an electron in caesium can have an n value from 1 to 6. What does the principal quantum number describegrant county wv deed search.
Leave a Reply Cancel reply. The first quantum number describes the electron shell or energy level of an atom. Energy n angular momentum ℓ magnetic moment mℓ and spin ms.
The first quantum number describes the electron shell or energy level of an atom. Energy n angular momentum ℓ magnetic moment m ℓ and spin m s. Required fields are marked.
What do each of the quantum numbers mean. The first quantum number describes the electron shell or. The first quantum number describes the electron shell or energy level of an atom.
To completely describe an electron in an atom four quantum numbers are needed. This explains why n can not be 0 or any negative integer because there exists no atoms with zero or a negative amount of energy levelsprincipal shells. To completely describe an electron in an atom four quantum numbers are needed.
The shape of the orbital. Chemistry questions and answers. The principal quantum number n describes the energy of an electron and the most probable distance of the electron from the nucleus.
To completely describe an electron in an atom four quantum numbers are needed. Nlmlms n represents the energy level of the electron where n 1234. The first quantum number describes the electron shell or energy level of an atom.
In other words it refers to the size of the orbital and the energy level an electron is placed in. To completely describe an electron in an atom four quantum numbers are needed. The quantum numbers are represented as.
It is the angular momentum quantum number andrefers to the shape of the orbital. Which orbital is being occupied C. The first three quantum numbers are the principal quantum number the azimuthal quantum number and the magnetic quantum number.
The principal quantum number denotes the energy shell level of the electron. By signing up youll get thousands of step-by-step solutions to your homework questions. The energy level of the electron.
To completely describe an electron in an atom four quantum numbers are needed. Which orbital is being occupied C. The number of subshells or l describes the shape of the orbital.
What does the first quantum number n describe. The value of n ranges from 1 to the shell containing the outermost electron of that atom. Energy n angular momentum ℓ magnetic moment mℓ and spin ms.
It refers to what energy level it is and will be onegreater than the number of nodes in the orbital. The spin that the electron has B. The first quantum number describes the electron shell or energy level of an atom.
To completely describe an electron in an atom four quantum numbers are needed. That is if n 2 there are two values of l given by 0 and 1. What does the fourth quantum number tell you about.
It is the first number and identifies the principle energy level - it indicates the average or most likely distance from the nucleus where the electron resides - positive integers only. The angular momentum quantum number l tells us the shape of the orbitals. The first quantum number describes the electron shell or energy level of an atom.
What does the first quantum number n describe. L 0 s-orbital. Quantum numbers are values that describe the energy or energetic state of an atoms.
The Principal Quantum Number n The first principal shell is also called the ground state or lowest energy state. The spin of the electron. 4 rows To completely describe an electron in an atom four quantum numbers are needed.
Energy n angular momentum ℓ magnetic moment m ℓ and spin m s. Energy n angular momentum ℓ magnetic moment mℓ and spin ms. The values of l depend on the value of the principal quantum number n.
What does the principal quantum number n describe. For a given value of n l has possible integral values from 0 to n - 1. What does the first quantum number n describe.
The spin that the electron has B. The first quantum number describes the electron shell or energy level of an atom. It is the principle quantumnumber.
The value of n ranges from 1 to the shell containing the outermost electron of that atom. It refers to what energy level it is and will be one greater than the number of nodes in the orbital. N is the first quantum number.
L determines what orbital shape it is where l 012n 1. The type of orbital the electron is in D. Your email address will not be published.
N also indicates the row on the periodic table. What do four quantum numbers describe about an electron. If n 3 there are three values of.
Ml is the third quantum number. Energy n angular momentum ℓ magnetic moment m ℓ and spin m s. What is the principle quantum number n.
It is the principle quantum number.

Quantum Numbers Introduction To Chemistry

Electron Configuration Tricks Ions Shortcuts Chemistry Education Electron Configuration Chemistry Classroom

Light And The Modern Atom Teaching Chemistry Chemistry Education Physics And Mathematics
0 Response to "What Does the First Quantum Number N Describe"
Post a Comment